Cryoadipolysis as a non-invasive procedure for the removal of adipose tissue was first used in 2010 after the
FDA approved a cryolipolysis device to reduce fat (K080521).
Many investigations, both in vitro[1] and on animals,[2] had
been conducted in the past to verify its efficacy and safety.[3]
Human clinical trials later confirmed that it was a safe technology with minimum pain and fast recovery.[4]
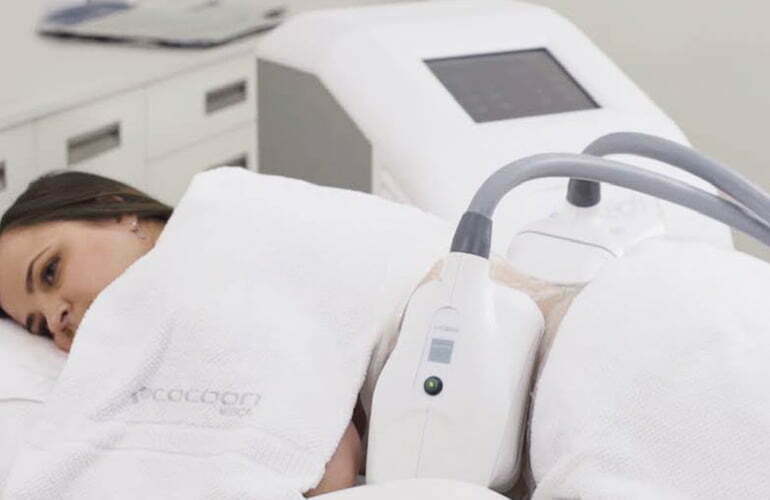
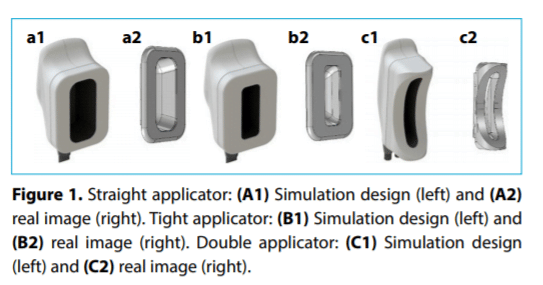
Within the development framework of new Cooltech Define® cryoadipolysis applicators, multiphysics simulation
software has proven to be a useful tool, since it offers the
possibility to virtually study how prototypes would behave
in a real-life situation through a model that includes the resolution of physical equations by finite elements calculation methods. This has already been verified in the previous
development of Cooltech® applicators.[4–6]
The main objective of this study was to evaluate the cooling efficacy of the Cooltech Define® applicators through
numerical simulations, and show their superiority to the
Cooltech® applicators, which implies greater affectation
of the fat inside of them, and therefore, it should reduce
fat percentage more effectively. Another objective was to
show that the design of the applicators can be optimized
using a merit parameter, based on the geometrical design
of the applicators. Finally, this study also showed the importance of considering the biological heat in the simulations and the need to perform clinical studies to determine the ischemia associated with each applicator